Autism
Projects: Kidney Analysis
The basic premise of the Brimstone Theory
is simple: disturbed metabolism of the oxides of sulfur cause autism.
In particular, sulfite is not properly processed resulting in low
levels of beneficial sulfate in blood. The kidney is responsible for
recycling sulfate but is compromised within autism, further
complicating matters. A diet rich in sulfate may be
preventative. The
problem is easy to correct by adding sulfate to water or switching to
sulfate rich bottled water. But first, we need to prove the theory is
correct. That was one goal of our study of the kidney, which is
summarized
below from a paper published in June of 2023 by the Biomedical
Journal
of Scientific and Technical Research.
Autism
and Renal Sulfate Transport
Abstract: Sulfate
is an important nutrient and enzyme cofactor. Blood sulfate is
depressed for individuals with autism, partly due to poor resorption
in the kidney. We model the kidney nephron using simple mathematics
and examine flowrates, concentrations and resorption along the length
of the proximal tubule. Assuming
constant resorption, NaS1 transport protein density is examined.
Blood levels of sulfite and thiosulfate inhibitors are increased to
show their influence on neurotypicals. Then blood sulfate is varied
to show how inhibitor levels may be decreased, potentially resulting
in symptom relief and improvement of overall health for those on the
spectrum. Expression of the NaS1 transport protein is
linked to vitamin D and estrogen chemistry, suggesting feedback
mechanisms for sulfate homeostasis. Finally,
sulfate supplementation and sulfite avoidance are discussed as
potential strategies for both the prevention and treatment of autism.
Introduction: Autism Spectrum
Disorders (ASD) affect social interaction, communication, behavior and
the senses. In the United States, the prevalence is 1 in 54 for all
children and 1 in 34 for boys based on data from the Centers for
Disease Control and Prevention. One characteristic of autism is
depressed resorption of sulfate in the kidney leading to high levels in
urine and low levels in blood. In this paper, we model the kidney using
simple mathematics to examine sulfate flowrates and concentrations.
Then the model is used to investigate sulfate inhibitors, sulfate
regulation and possible steps to correct imbalances.
An important feature of autism is dysfunctional sulfur metabolism. In
particular, the oxides of sulfur are implicated: sulfite, thiosulfate
and sulfate. Sulfate may be ingested directly or it may be converted
from the amino acid methionine by a series of enzymes including sulfite
oxidase. An English study reports the urine of those with autism
contains 50 times the sulfite, 7 times the thiosulfate and double the
sulfate of neurotypicals. An Arizona study found depressed levels of
blood sulfate in those with autism, only 35% of normal in the case of
free sulfate. And a French study of nasal stem cells found 91% of those
with autism had decreased expression of genes (MOCOS and AOX) within
the molybdenum cofactor pathway. This pathway is responsible for
several important enzymes including sulfite oxidase. There are 5
upstream genes (MOCS1, MOCS2, MOCS3, NFS1 and GPHN) in this pathway,
requiring several cofactors including bioactive vitamin B6 (PLP).
Interference with of any of these elements will impair sulfite oxidase
enzyme and depress the conversion of sulfite to sulfate as indicated
above.
Sulfate within the kidney filtrate is returned to the blood via
resorption through proximal tubule membrane cells. This is facilitated
by two transport proteins: NaS1 (SLC13A1) sodium-sulfate co-transporter
located at the brush border membrane and SAT1 (SLC26A1) anion exchanger
located at the basolateral membrane. NaS1 moves sulfate from nephron
lumen into kidney membrane cells and SAT1 moves sulfate from membrane
cells back into the bloodstream. When operating properly, they help to
maintain sulfate blood levels within a healthy range. For those with
autism, kidney resorption is partially blocked resulting in urine
levels that are double normal and blood levels that are one third
normal as reported above.
Simple
Model of the Kidney Nephron: The human kidney pair contains
approximately one million small tubes called nephrons. As blood passes
through tiny pores upon entry, red and white cells are blocked and only
plasma passes into the nephron. As this filtrate moves along the small
tubes, nutrients are returned to the bloodstream while waste and toxins
flow into the urine. The front section of each tube is called the
proximal tubule and this region is responsible for the resorption of
65% of the general filtrate and nearly all of the sulfate. The inner
brush border membrane of the proximal tubule includes NaS1 transport
proteins that move sulfate from the filtrate into the cytoplasm of the
cells lining the tube. The outer basolateral membrane includes SAT1
transport proteins which complete the task by moving sulfate from
cytoplasm back into the blood. It is generally assumed that NaS1
proteins form the rate limiting step for sulfate transport, therefore
this study will consider only flowrates and kinetics for NaS1 transport
proteins.
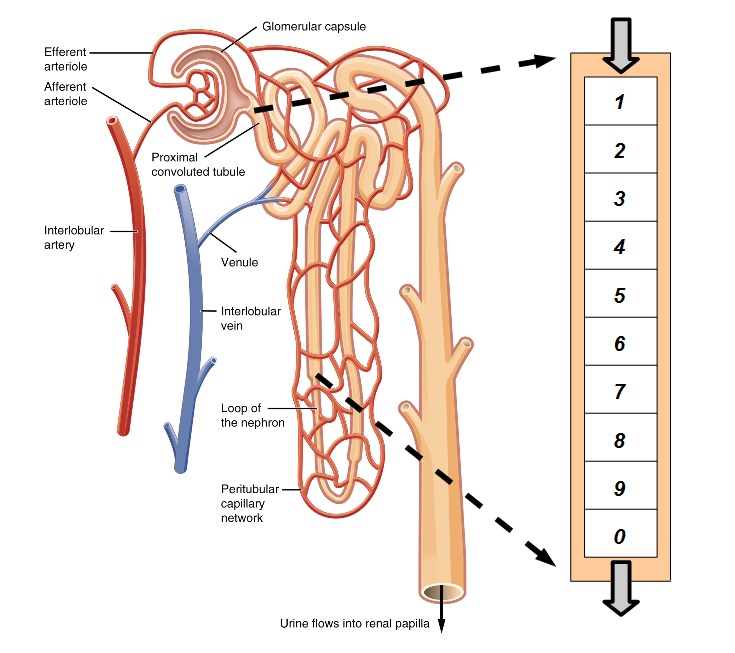
Figure 1. Simple Model of the Kidney Nephron
As shown in Figure 1, we model the kidney nephron as a tube,
beginning with the proximal tubule divided into 10 segments which are
followed by an undifferentiated remainder. As filtrate flows down the
tube, the concentration of sulfate and its inhibitors varies as they
are reabsorbed along with water and other chemicals. The flowrate of
chemicals in the filtrate can be specified at the entry and exit points
by multiplying the appropriate concentrations by the flowrate of water.
For a typical pair of human kidneys, the flowrate of the filtrate
(which is mostly water) is 180L/day at the bloodstream entry and about
1.4 L/day at the urine exit.
Noting that 65% of the filtrate is reabsorbed in the proximal tubules
along with nearly 100% of sulfate, we can make a few assumptions. Since
the filtrate is mostly water, the flowrate of water at the end of all
the proximal tubules would be approximately 35% of the blood entry
flowrate or 63L/day. Whereas for sulfate, the flowrate at the end of
the proximal tubules would be the same as the urine flowrate. And the
same would apply to the competitive inhibitors or their combination.
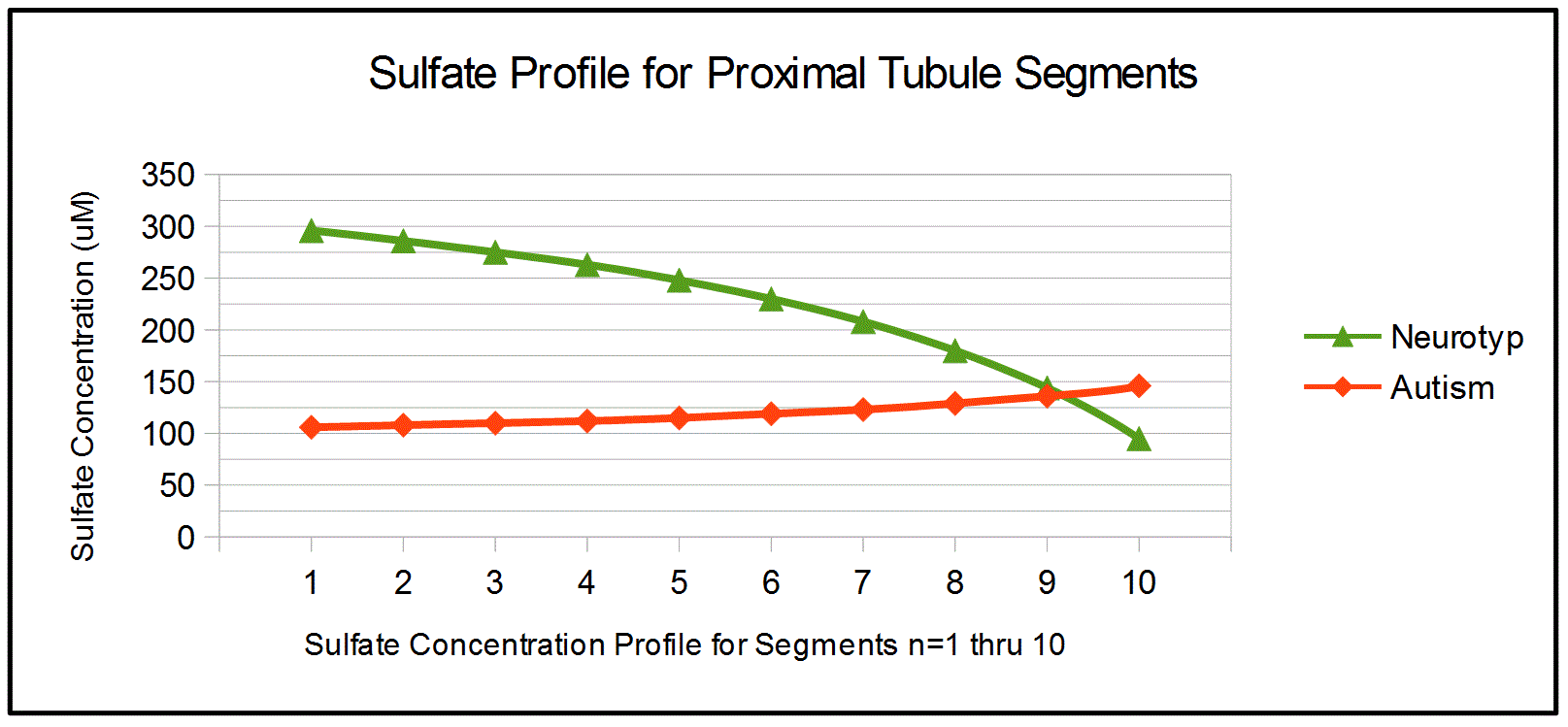
Figure 2 plots sulfate concentrations for segments n=1 to n=10. For
neurotypicals, sulfate concentrations drop as water is removed more
slowly than sulfate in the proximal tubule. Within autism, this effect
is countered by the higher levels of sulfate in urine.
Figure 2. Sulfate Concentration for
Nephron Segments
Discussion: The concentration
profiles in Figure 2 hightlight the differences in kidney function
between neurotypicals and those with autism. It seems logical to assume
these differences result from variations in the density of sulfate
transporters embedded in the surface of the tubule membrane. And this
suggests that the expression of NaS1 transport proteins may play an
important role in sulfate regulation. If the expression of NaS1 can be
properly linked to sulfate levels, a regulatory feedback loop may be
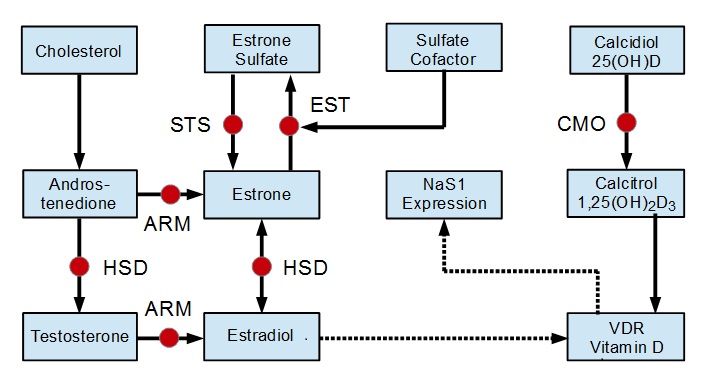
established. Pathways relevant to this discussion are shown in Figure 3
that follows.
Figure 3. Simplified Metabolic
Pathways for Sulfate Regulation
An Australian genetic analysis of NaS1 has identified a Vitamin D
responsive element in the promoter region of the gene. And a study of
VDR knockout mice with diminished vitamin D receptor expression showed
urinary sulfate increased by 42% while blood serum sulfate decreased by
50%. These studies confirm that repression of either vitamin D or its
receptor interferes with the NaS1 transporter causing sulfate
resorption to decrease. Studies of pregnant women in Sweden have noted
Vitamin D (25OHD) deficiency increased autism risk by a factor of 1.58.
On the other hand, the Arizona study of blood sulfate previously
referenced also tracked vitamins and minerals. For vitamin D, there was
very little difference between neurotypicals and children with autism.
In fact, those on the spectrum measured about 2% higher. Perhaps this
is a clue that the vitamin D receptor (VDR) may be a more likely
candidate for regulation of NaS1 and sulfate.
VDR expression is regulated by the hormone estrogen. Estrogen is a
family name for several similar chemicals including estrone and
estradiol which are the most abundant. Estrone and estradiol may
interconvert as needed. Estrone may be removed by estrone
sulfotransferase (EST) to form a sulfate and returned via the enzyme
steroid sulfatase (STS). Estrone sulfate acts as a reserve pool
allowing regulation of overall estrogen. An important piece of this
process is the cofactor sulfate. Without sufficient sulfate, estrone
removal via EST is diminished which keeps overall estrogen levels high.
This connection to sulfate completes a feedback loop that may play an
important part in sulfate regulation.
Regulation
of Sulfate via Negative Feedback
Sulfate blood levels drop.
EST is starved for its sulfate cofactor.
Estrone rises which up-regulates VDR expression.
This creates NaS1 proteins that bolster renal sulfate resorption.
Increased resorption raises blood levels of sulfate to maintain
homeostasis.
The feedback loop described above may offer insight into sulfate
homeostasis which maintains normal serum concentrations in the vicinity
of 300uM. Simply put, sulfate levels drop and this leads to enhanced
NaS1 expression with increased sulfate resorption. However, simple
logic suggests that regulatory feedback within autism must be
compromised if overall sulfate resorption is so strongly depressed. For
those on the spectrum, average values of sulfate, maximum velocities
and protein density are all depressed. Regulatory feedback would try to
correct this but fails. Why?
The proposed feedback loop relies on estrone to adjust the density of
sulfate transport proteins. When sulfate falls, EST reduces the
sulfation of estrone and estrone levels should rise. Of course, this
assumes that other paths also feeding estrone remain unaffected. A
recent Chinese study of steroid sulfatase (STS) has shown sulfite to be
an inhibitor of this enzyme. If sulfite inhbition is significant, STS
conversion of estrone sulfate back to estrone would be reduced. This
negates increases in estrone required by the sulfate feedback loop. Our
analysis has estimated autism blood sulfite would result in a 30%
inhibition of STS, disturbing regulatory feedback for those on the
autism spectrum.
Conclusion: Metabolism of sulfur is quite disturbed within
autism. Sulfite in urine is 50 times normal while thiosulfate is
increased 7 fold. Free sulfate is double in urine and only one third
normal in blood. Dysfunctional levels of these oxides of sulfur may be
explained by abnormalities within the molybdenum cofactor pathway,
which are present in 9 out of 10 children with autism. In turn, these
pathway abnormalities interfere with the creation of sulfite oxidase
enzyme, necessary for the conversion of sulfite into sulfate. Low
sulfate in conjunction with high sulfite and thiosulfate reduces renal
resorption, further lowering blood sulfate. Sulfate regulation would
help to correct this shortfall but may be compromised by inadequate
vitamin D or its receptor (VDR). Higher testosterone and lower estrogen
typical in males would reduce the expression of VDR, possibly
explaining why boys are more strongly affected by autism than girls.
In this paper, incomplete published data was augmented by estimates to
more fully characterize blood levels and transport properties of
sulfate in the kidney. A simple model of the kidney nephron was built
assuming constant sulfate resorption along the length of the proximal
tubule. Flowrates and concentrations were calculated and plotted,
demonstrating how elevated sulfite and thiosulfate could interfere with
renal sulfate resorption even in neurotypicals. A feedback mechanism
was proposed to explain the regulation of sulfate via vitamin D and
estrogen chemistry. It is hoped that this study expands the
understanding of sulfur metabolism, leading to autism strategies that
increase sulfate, lower sulfite, reduce prevalence and improve
treatment. To view the full article,
click on the link below.
|

|
Hosted by
Rybett Controls
|

|